One Of The Best Tips About How To Tell If A Reaction Is Endothermic Or Exothermic

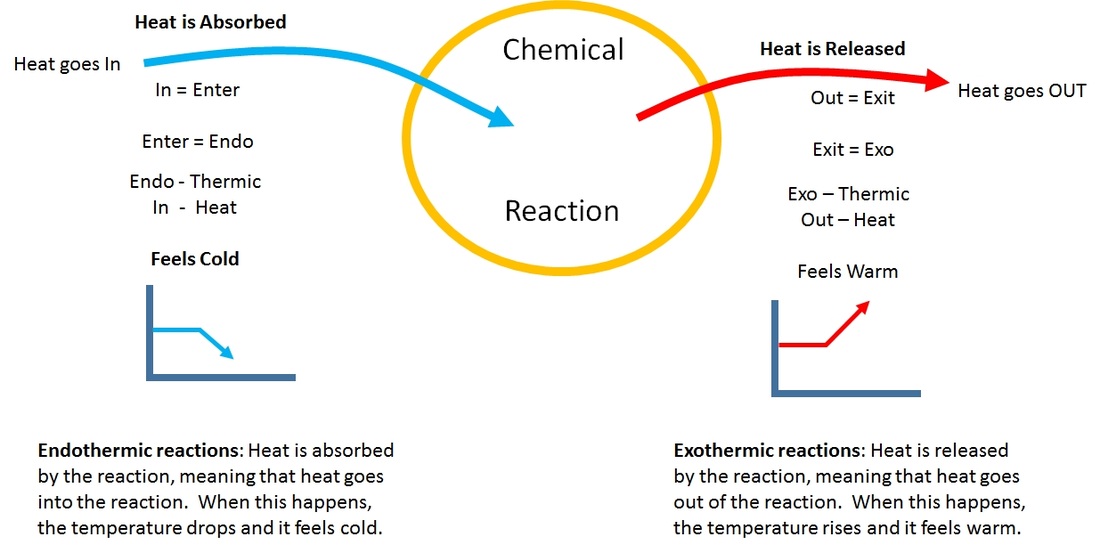
Endothermic reactions require energy, so energy is a reactant.
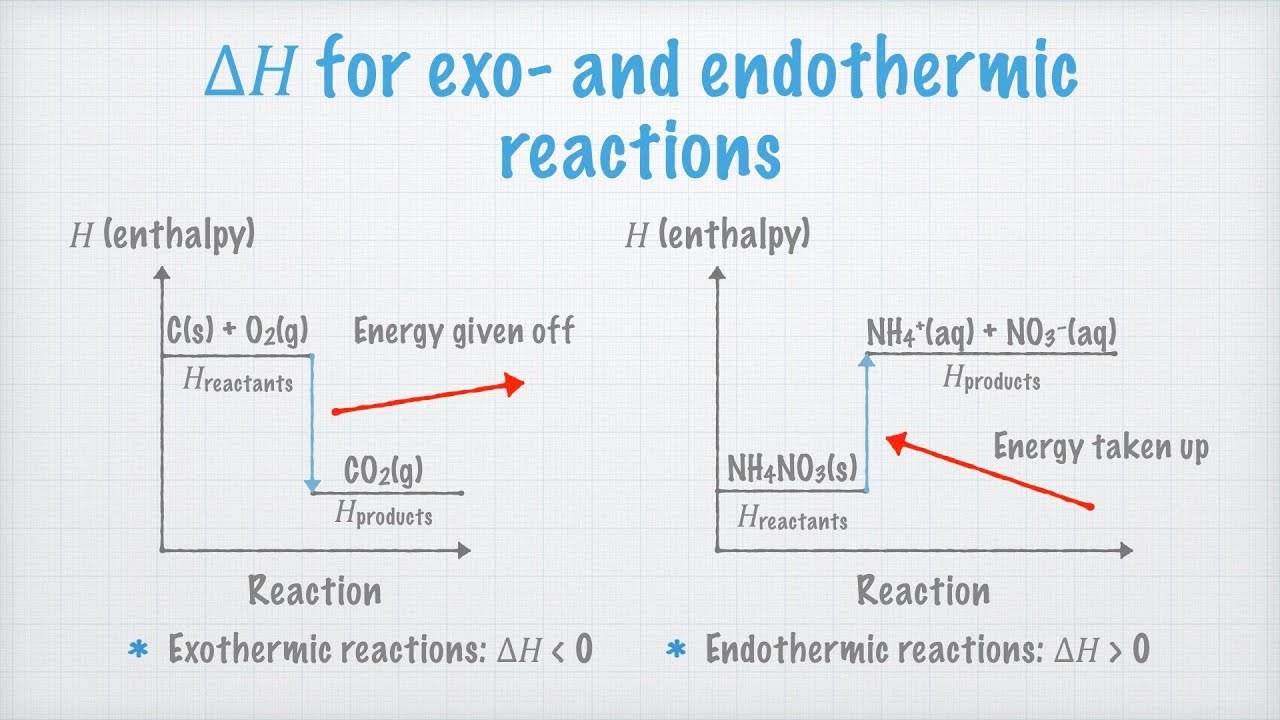
How to tell if a reaction is endothermic or exothermic. But is it possible without. If δh is negative, the process releases heat to the surroundings. Determining if a reaction is endothermic or exothermic equations and.
When a chemical reaction happens, energy is transferred to or from the surroundings. We can use the law of conservation of energy to. Result how do you tell if a reaction is exothermic or endothermic?
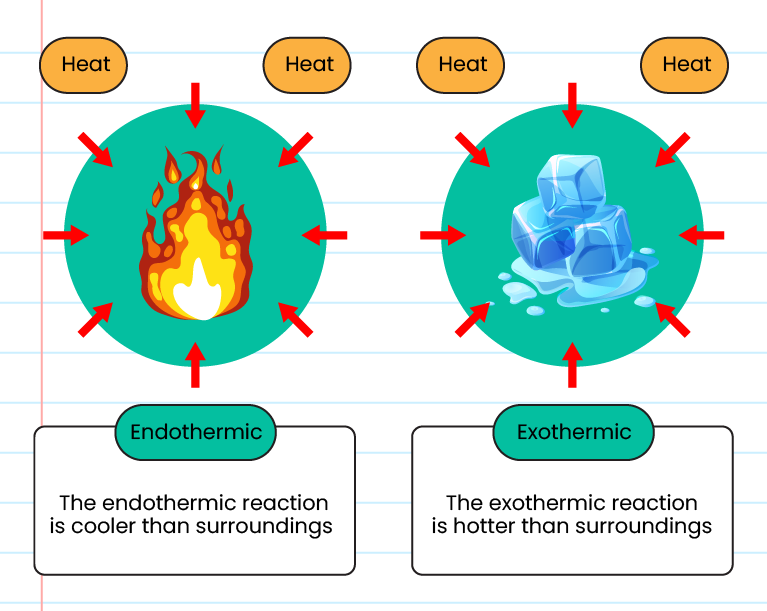
Decomposition reactions can be exothermic or endothermic, depending on the chemical energy of the. What is an endothermic reaction? Heat flows from the surroundings to the system.
Result how to judge if a reaction is exothermic or endothermic. Modified 8 years, 5 months ago. Tes paid licencehow can i reuse this?.
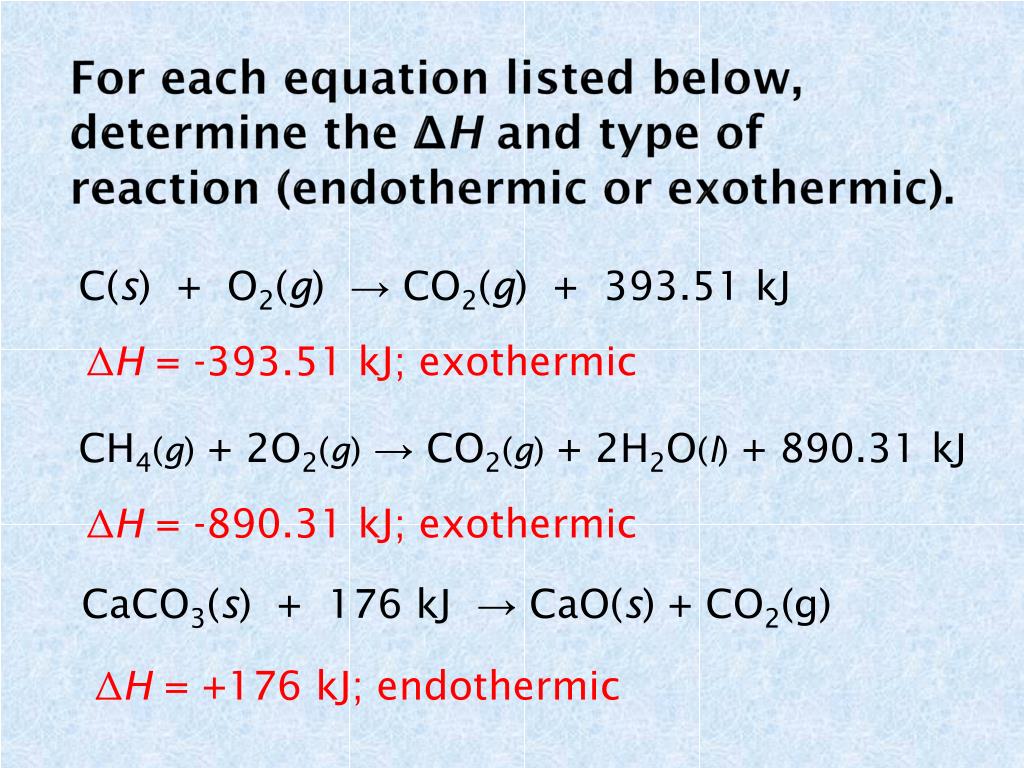
Result endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Result if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. See examples of exothermic and endothermic.
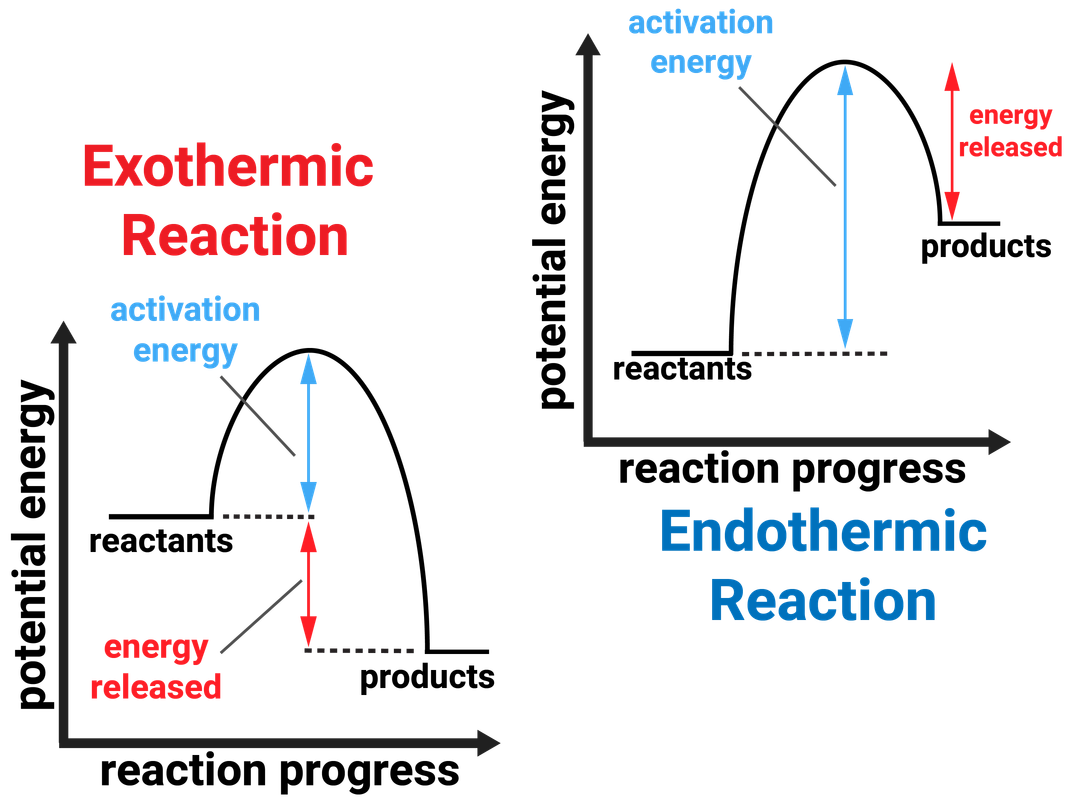
Result endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Result learn how to classify chemical reactions as exothermic or endothermic by analysing the changes in chemical bonding. Result for a general reaction, search for standard enthalpy of formation of all reactants and products, multiply the values by stoichiometric coefficients and.
An endothermic reaction or process takes place when the system absorbs heat energy from the surrounding. A good example of an endothermic. Result the endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat.
In this investigation, students classify chemical reactions as exothermic or endothermic. Result specifically looking at endothermic and exothermic reactions within the topics chemical and energy changes. In a chemical reaction, if the sum of the enthalpies of all the reactants is greater than the sum.
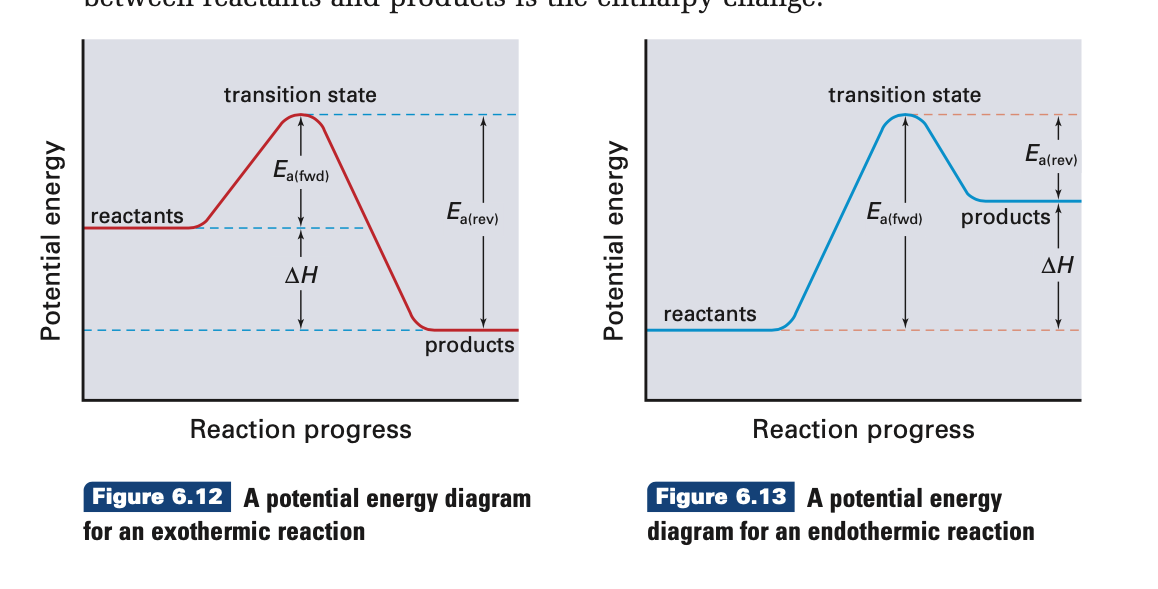
Result endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Determine if the net flow of energy (heat) is into or out of the process. Result i already know the way to determine whether if the given reaction is exothermic or endothermic by the enthalpy values.








:max_bytes(150000):strip_icc()/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)